TGI MH Friday, April 9, 2021
Good morning and welcome to another addition of TGI MH Friday. Thanks for your patience this week while I sorted out some health issues. This morning’s issue of PAAD was written by Robbie Shaw from the University of Wisconsin. He just started his first run of being a hotline consultant and I’m mentoring him. He received a great case that will be the subject for us today.

Our patient is a 3-year-old female with history of multiple congenital anomalies and arthrogryposis, undergoing repair of a tethered cord. She had uneventful previous anesthetics without any complication, including multiple exposures to triggering agents for MH. For this case she had sevoflurane, followed by rocuronium for intubation. Immediately following intubation, she felt rigid with rise in EtCO2 from baseline of 40s to mid 60s. Hand ventilation was maintained without changes in lung compliance or minute ventilation. The team then stopped the sevo and the patient was transitioned to TIVA for the remainder of the case. The patient was never tachycardic, hypertensive, or febrile. EtCO2 improved (32 at time of consultation) with normal ventilatory parameters after discontinuation of sevoflurane. The rest of the procedure continued uneventfully, and the patient was admitted postoperatively for further recovery. A genetics consultation was suggested to evaluate underlying myopathy or RYR1 receptor mutation.
Could this have been MH? What is arthrogryposis and its relationship to MH susceptibility?
Arthrogryposis (Greek, arthro:joint, gryp:curved) is a descriptive term for congenital contractures that affect two or more different areas of the body. The finding is present in 1 in 3,000 births, representing over 400 specific conditions, with 150 of these having respective gene alterations. The etiology of arthrogryposis is thought to be a result of fetal akinesia, with a direct correlation between onset of decreased fetal movement and contracture severity. This decrease in movement has been postulated to cause an increase in connective tissue around joints, disuse of muscles around the joint, and abnormal joint surfaces.
Fetal akinesia can result from a variety of causes. Neuromuscular disorders are of most interest in evaluating a patient with arthrogryposis and MH susceptibility. Congenital myopathies can be associated with arthrogryposis. These can be a result of mutations in genes encoding the ryanodine receptor (RYR1), fetal myosin heavy chain (MYH3), and skeletal-muscle thin filament proteins. Of specific concern to the pediatric anesthesia provider is the potential for RYR1 mutations, which have been reported in the literature, raising the risk for MH susceptibility.
There is no direct correlation between arthrogryposis and MH. A 1986 paper examined 398 anesthetics in 67 patients with arthrogryposis over a 32-year period (1952 to 1984), with multiple exposures to MH triggering agents (mostly halothane), and found no evidence of MH. A 1991 case series involved 2 patients with a hypermetabolic response (hyperthermia, tachycardia, hypercapnia) to MH triggering agents. The authors genetically tested one patient’s parents after presumed MH episode, and both were normal (the child was too young for contracture biopsy). The authors surmised that while patients with arthrogryposis can have a hypermetabolic response to MH triggering agents, it is distinct from MH.
These cases are difficult to know what to do…remember there is no bedside test for MH. The team did a great job assuming MH and treating accordingly. Her reaction to sevoflurane along with improvement after its discontinuation warrant further evaluation, especially genetic testing for RYR1 gene mutations.
We hope you have a great spring weekend, and wishing all those on call the chance to go outside for some sunshine.
TGI MH Friday, April 2, 2021
In today’s issue of TGI MH Friday, I’ll summarize a recent update from the European Malignant Hyperthermia Group on how to manage known or suspected MH susceptible patients when they present for surgery. There’s nothing new or surprising about their recommendations, but it’s always good to review once in a while (especially on a Friday when our brains are slipping into weekend mode).
Rüffert et al. used the Delphi method (i.e., the formalized consensus process of successive iterations of expert opinion when not enough scientific evidence is available) to come up with a variety of recommendations for optimal preparation and management of these patients. The paper and the figure that I’ve reproduced below goes into more detail, but here are their core recommendations:

· Pretreatment with dantrolene is not indicated.
· Prepare the anesthesia machine by either using a machine that has never been exposed to volatile anesthetics or flush the gases out using at least 10 mL/min according to the manufacturer or expert recommendations (Table 2 in the paper provides specific details).
· Use a brand-new anesthesia circuit and soda lime prior to machine flushing.
· If available, attach charcoal filters to the inspiratory and expiratory limbs of the anesthesia circuit and flush for 2-3 minutes.
· Remove the vaporizers. Some places will routinely put tape over them to remind the anesthesia provider not to use them, but this isn’t foolproof. I heard of a case where the anesthesia tech was urgently called away while in the middle of doing this, forgot to return to finish the taping, and the MHS child accidently received sevoflurane for a short time until the team realized it (luckily did well).
· After charcoal filters are placed and breathing circuit and soda-lime canister are changed, usual fresh gas flows can be used with a minimum of 1 L/min.
· Use only trigger-free anesthetics, essentially anything except volatile anesthetics and succinylcholine. Anything else you can think of is safe.
· No additional monitoring is required, over and above the usual monitors chosen for that patient.
· No preoperative or postoperative labs are necessary, other than those the patient was going to get anyway.
· Outpatient anesthesia is allowed. The authors here do not distinguish between outpatient anesthesia in a hospital facility and a free-standing surgi-center (ASF). To me, it makes sense that known MHS patients given a non-triggering technique in a free-standing center would be inherently safer. After all, you already know they are MHS. It’s the patients that you don’t know are MHS that are at risk if given a volatile anesthetic far from a tertiary care center. But, many facilities, including my own, specifically exclude MHS patients from having elective surgery at the ASF, mainly for reasons of throughput. On a busy day with tubes and tonsils, the last thing you want to do is to stop the momentum by having to prep the machine and make up the propofol infusion pump.
· Recovery and discharge criteria are the same as for any other non-MH susceptible patient.
I hope everyone has a relaxing and baseball-filled weekend (#LGM) and for those of you taking the weekend calls, hope you get some down time to watch your favorite team. And if you love reading PAAD every day, send to all your friends.
Throwback Thursday, April 1, 2021
Before we get to today’s Throwback Thursday article, I have some exciting news that ain’t no April Fools! At the beginning of March, I began collecting voluntary donations from PAAD readers, and I’m happy to announce that stunningly, in one month you have contributed $2,718! That entire amount has been donated to the SPA Patient Safety Education & Research Fund, and I will forward to them the names of all paid subscribers. (Substack, the provider of this subscription platform and processor of payments keeps 10%.)
And now, on to today’s Throwback Thursday…
How many PAAD readers have ever read the package insert (i.e., “label”) for nitrous oxide? Don’t ponder that for too long because it was a trick question. There is none. That’s because nitrous was in use for many decades before the FDA ever existed. In 1844, Horace Wells, a discontented dentist, made a fool of himself on the stage of a ‘Grand Exhibition’ after inhaling nitrous oxide. That same evening, he observed that another participant had injured his leg while dancing on the stage and felt no pain until the effects of the nitrous oxide had worn off. When Wells later inhaled it and had his former student John Riggs remove a tooth, he exclaimed ‘It is the greatest discovery ever made! I didn’t feel it so much as the prick of a pin!’.

Here we are, 177 years later, and nitrous oxide is still a bit of a mystery. After all this time, its safety seems unimpeachable. But there are continuing controversies whether it should be part of our pediatric anesthesia armamentarium. Some of us love it, and some do not. Some, like myself, use it sparingly (e.g., only during induction to increase the speed of unconsciousness) and then turn it off. So, in today’s Throwback Thursday, I want to highlight the possible complications of N2O using a paper published in 1992 that caused quite a stir at that time.
In brief, Rowland et al. sent questionnaires to 7,000 female dental assistants, ages 18 to 39, and through follow-up telephone interviews, determined that women exposed to high levels of nitrous oxide were significantly less fertile than women who were unexposed or exposed to lower levels of N2O. It wasn’t a great epidemiological study, as demonstrated by the fact that the effect was evident in only 19 women with 5 or more hours of exposure per week, but it was still jarring for all anesthesia providers.
Nitrous oxide inactivates vitamin B12 and thus, inhibits the activity of methionine synthetase. This mechanism may have played a role in the decreased fertility of the dental assistants. But that property has also been implicated in causing complications in anesthetized children. Dr. Kirk Hogan, a passionate opponent of the use of N2O has stated:
“94% of teenagers with broadly distributed, decreased white matter integrity and volume on magnetic resonance imaging of their brains inhaled nitrous oxide for over an hour during surgery and anesthesia in their first year of life…and 88% of children between the ages of 5 and 18 yr with lower gray matter density in the occipital cortex and cerebellum inhaled nitrous oxide during surgery and anesthesia before their fourth birthday. Nitrous oxide is the only inhalational anesthetic that causes demyelination, cerebral atrophy, and loss of developmental milestones in a susceptible child after use in clinical concentrations and durations. One hour of nitrous oxide administration is sufficient to inactivate methionine synthase by oxidation of cobalt in its vitamin B12 cofactor. Up to 20% of infants and children in North America express one or more alleles that impair the activity of enzymes in single carbon pathways in which methionine synthase is the pivotal participant. Up to 25% of infants and children before the age of 10 yr are deficient in vitamin B12. Accordingly, up to 5% of infants have both an inborn and an acquired deficiency of vitamin B12 at the time they are anesthetized with nitrous oxide.” (See the link above for all references)
I’ll add a few more examples of the hazards of N2O use: An 8-month-old male infant with unrecognized nutritional vitamin B12 deficiency underwent orchiopexy under general anesthesia that included N2O. Six days later, the infant presented with fever, progressive lethargy, athetoid movements, and bone marrow failure. The infant recovered after several days of vitamin B12 supplementation. The authors speculated that exposure to N2O exacerbated this infant’s condition, and precipitated the neurological deterioration and bone marrow failure.
A 4-month-old female developed neurological deterioration and severe metabolic acidosis 3 weeks following elective craniosynostosis repair under general anesthesia that included N2O. Subsequent investigation revealed a nutritional vitamin B12 deficiency (of unknown etiology) that gradually resolved following exogenous supplementation.
Lastly, a case report described the neurologic deterioration and death of a 3-month-old infant anesthetized twice with N2O prior to establishing a diagnosis of 5,10-methylenetetrahydrofolate reductase deficiency, a rare autosomal recessive disorder.
It is unlikely that the therapeutic margin of nitrous oxide will ever become small enough to preclude its use altogether, but convincing arguments for its continued inclusion in pediatric anesthesia are tough to find.
Enjoying these PAADs? Please forward to your colleagues and friends throughout the world!
Wednesday, March 31, 2021
I’ve been thinking lately that I haven’t devoted enough attention in PAAD to pediatric pain issues. And then I noticed this MGH clinicopathologic conference (CPC) from last week’s NEJM. I’ve been reading these CPCs since I was a med student in the 1980s, and although very few are of interest to practitioners of pediatric anesthesia, I take notice of the ones that involve children, and there’s still some that are very enjoyable to read, even if they aren’t directly applicable to our practice. But this one involves a case that sooner or later, many of us will encounter.
In brief, the authors describe a teenager with a 17-month history of headaches after an ATV accident, which were then exacerbated after a soccer injury. Over that time interval he was administered a slew of medications for stress or migraine headaches, but nothing was effective. Fast forward those 17 months, when he presented to an outside hospital (and then eventually transferred to MGH) with severe headache, abdominal pain, and a hypertensive crisis, with BP in the outside hospital recorded at 239/162 and a HR of 160 bpm! His WBC was 22K, and his subsequent ECG showed an ectopic atrial rhythm with frequent premature ventricular contractions at a rate of 66 beats per minute.
SPOILER ALERT! If you enjoy trying to figure out the diagnosis and want to read the article first, then do not read this any further.
The team at the outside hospital recognized this as a true hypertensive emergency and started the patient on hydromorphone, morphine, ondansetron, metoclopramide, nitroglycerin, labetalol, and esmolol (it’s not detailed in the report the order or magnitude of these drug administrations). His blood pressure was lowered to a stable level, and then he was transferred to MGH. On arrival to MGH the esmolol was discontinued, and the team there apparently made the diagnosis of a pheochromocytoma fairly easily using abdominal ultrasound and confirmation by blood and urine catecholamine levels.
Those of you, like me, who do one of these cases every few years will be familiar with the stress and anxiety associated with the concern for intraoperative fluid management and blood pressure control. In preparation for surgery, the PICU team at MGH focused on fluid resuscitation and controlling the patient’s BP with a nicardipine infusion. Once the diagnosis of a pheo was confirmed, he was started on phenoxybenzamine, a long-acting alpha-blocker. The authors discuss the hazards of using only beta-blockade, which might result in unopposed alpha receptor agonism, worsening the hypertension. They mention that beta blockade would be considered only in the setting of adequate alpha blockade and a high heart rate, which happened in their patient, who was then administered labetalol, a mixed alpha- and beta-blocker. The patient eventually underwent an uneventful surgical resection 13 days later, which underscores the importance of preoperative medical management of these patients.
I took a look at the section on pheo management in Uptodate.com and there seems to be a variety of ways to manage these patients preoperatively, with several choices for alpha blockade. One of the authors mentions residual postoperative hypotension from the lasting effects of phenoxybenzamine. Whatever regimen your ICU colleagues choose, the most salient teaching points here are to ensure volume resuscitation, consistent control of catecholamine-induced hypertension, and a well-functioning arterial line before bringing the patient to the OR for surgical resection.
Tuesday, March 30, 2021
OK, so the “journal” Best Practice & Research Clinical Anaesthesiology doesn’t present much in the way of original research, but this review article recently caught my eye. It’s well-written and up to date, so I highly recommend spending a spare 20 minutes with it. De Graff et al, from Sophia Children’s Hospital, University Medical Center Rotterdam, in the Netherlands, and Montreal Children’s Hospital, have given us a succinct update on pediatric anesthesia safety. It’s a little heavy with numbers in different studies, but the authors emphasize the most salient points: healthy children have virtually no risk, and of course, unhealthy children have a relatively high risk for intraoperative respiratory and cardiac critical events, and postoperative mortality. In this latter group, younger age, higher ASA-PS class status, cardiothoracic, and emergency, or multiple surgeries are common risk factors. So is provider experience.

I thought of this article while reading a great book recommended to me by John Fiadjoe, who is always on the cutting edge of ways to improve safety. The book is called Black Box Thinking, Marginal Gains and the Secrets of High Performance, by Matthew Syed. It consists of a compilation of case studies that focus on learning from failure. One of the most important chapters describes Team Sky, the British professional cycling team. They leaped from mediocre to world champions in just a few years. Their secret? Marginal gains. Minor tweaks here and there with the end result of improving so many tiny incremental components, that in the end, they add up to a winning combination. I thought of the typical low risk ASA 1 patient we take care of routinely. They always do well, but what marginal gains are still there for the taking? Turns out, there are plenty. Some that come easily to mind include less postoperative pain, less PONV, fewer corneal abrasions, and many more you already know and are described nicely by de Graff, but that we don’t always think about for every case we do.
The concept of marginal gain has not been talked about much in the anesthesia literature. But a Google Scholar search on the subject revealed a great editorial on the subject by Leng and Mariano in Regional Anesthesia & Pain Medicine, refuting a study that showed “only” marginal gains from performing TAP blocks in bariatric surgery patients. It’s short and also worth a read.
So, your homework for today: read the de Graff and Leng papers, and think about how you can achieve marginal gains in your patients, even if no one ever notices.
Medicolegal Monday, March 29, 2021
Over the years, I’ve been asked to review multiple lawsuits resulting from complications during pediatric airway management. After all, hypoxia resulting from critical respiratory events is the most important reason why children develop brain damage in the hospital setting. Invariably, these cases were not managed by anesthesia professionals, but rather by other types of healthcare providers (e.g., pediatricians, nurse practitioners, adult ER docs, etc.) tasked with emergency resuscitation of (mainly) infants in different settings, such as emergency rooms, NICUs, and labor & delivery suites. The one thing they all have in common is the inability to oxygenate, either because of technical difficulties with tracheal intubation, or failure to bag-mask ventilate adequately. So, I thought this would be a good opportunity to review six important lessons I’ve learned from evaluating these cases that can be adopted by even the most expert pediatric anesthesia providers:
1. Know PALS and the Neonatal Resuscitation Algorithm. Cold. Make sure your certificates never expire. Those certificates are good for two years, but each institution should perform simulations at least yearly for all their staff. In pretty much every case I’ve reviewed, the plaintiff’s attorney will ask the defendant about their certificate status at the time of the critical event that led to the complication. Even after you have renewed your certificates, make sure you always have a handy reference immediately available, in the form of an OR wall poster, pocket-sized handbook, or others. There is no reason why any of us has to memorize certain details of critical incident treatments, especially in the midst of a stressful crisis. The Society for Pediatric Anesthesia has a great app (Pedi Crisis) that can be found here. Take advantage of this valuable resource and download it now.
2. Know any of the many published Pediatric Difficult Airway Algorithms. Cold. Most of these algorithms are similar, with a few slight differences. Here’s one published by a multidisciplinary team at the Children’s Hospital of Philadelphia, and here’s the official ASA version, applicable to both adults and children. This should also be immediately available in every OR and other anesthetizing or critical care locations.
3. If hypoxia-induced bradycardia occurs, the drug of choice is not atropine or epinephrine, it’s oxygen. If oxygen is not transferred into the patient’s lungs the bradycardia will not resolve. In every case I’ve reviewed, the defendant has sworn that the patient remained bradycardic even though they were achieving adequate bag-mask ventilation. Unless the hypoxia has been so profound and of enough duration to cause cardiac ischemia, this cannot be the case. Even seasoned professionals may have difficulty discerning adequate lung inflation with bag-mask ventilation. Although there can be a lag between getting the oxygen to the brain/heart and resumption of normal sinus rhythm, one must always assume that bradycardia means hypoxia.
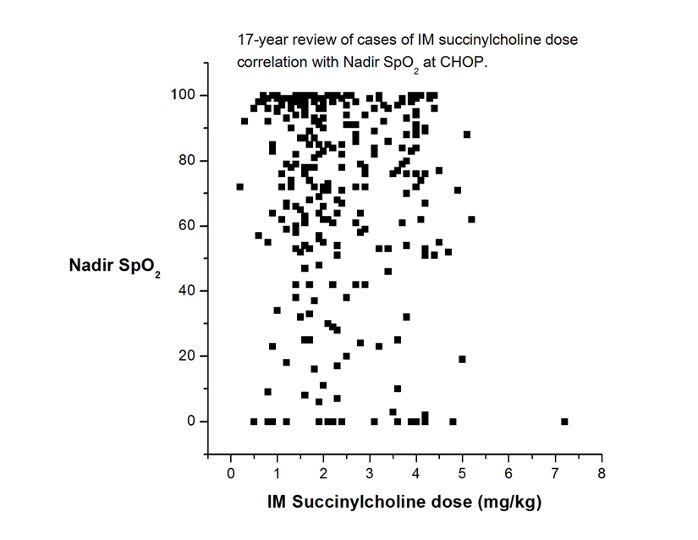
4. Use succinylcholine sooner rather than later. While I realize this statement will have its detractors, I’m convinced that succinylcholine has saved more lives and brain cells than any other intervention we have for life-threatening hypoxemia. Sure, it has side effects, but they are nearly always manageable and secondary to brain damage. If the patient already has an indwelling IV, very small amounts of succinylcholine (0.1 mg/kg) have been reported to be effective. If an IV has not been placed, IM succinylcholine is also effective. How much to give? The most often cited reference (Liu et al, 1981) recommends 4 mg/kg. A few years ago, one of our great fellows, Mike King, looked at our dose experience of IM succinylcholine at CHOP over 17 years. He generated this (unpublished) chart, which shows that most administrations were not as high as 4 mg/kg. But, the more you give, the faster it will work (BTW, those really low sats certainly look scary, but not one child in this time period was harmed, and I’ll bet anything that your children’s hospital has a very similar graph.)
5. Place your favorite supraglottic airway (SGA) sooner than later. Don’t wait because you think you can mask ventilate, and don’t perseverate trying to intubate. In our anesthesia world, this has become second nature to us. But surprisingly, it’s not yet become routine in other areas such as the NICU or delivery room. SGA placement is now included in the most current version of the PALS guidelines, but it is still absent in the most current neonatal resuscitation guidelines.
6. As we discussed in a previous Medicolegal Monday, think about tracheostomy sooner than later. In Complications, A Surgeon’s Notes on an Imperfect Science, Atul Gawande describes a horrific scene of a trauma victim in the ED who rapidly developed hypoxemia and required multiple attempts to secure the airway. Although he thought of tracheostomy at that moment, as the trauma resident responding to the scene, he was too shy to insist on it, and that indecisiveness contributed to a poor outcome.
And on that note, as always on Mondays,
“Let’s be careful out there!”
Saturday, March 27, 2021

Good morning…Spring has sprung in Philly, and, no matter who you root for, opening day is only 5 days away!
First and foremost, SHOUT-OUTS to PAAD’s newest Founding Member Subscribers: Drs. Robert Christensen, Daniela Damien, and Tara Hatat. THANK YOU! Your voluntary contributions to PAAD will be 100% donated to SPA’s Patient Safety, Education and Research Fund (PSERF) that supports junior investigators. I will be posting March proceeds and donations later this week.
ICYMI, here are the newsletters from the past week:
Medicolegal Monday March 22, 2021: What is off-label prescribing? Is it legal? Do we put ourselves at medicolegal risk for this practice?
Tuesday March 23, 2021: A death knell for liposomal bupivacaine?
Wednesday March 24, 2021: Does anesthesia affect babies’ brains? Where do we stand?
Throwback Thursday March 25, 2021: We welcome back distinguished Professor Myron Yaster. He discusses a classic paper on the origins of the way in which we dose neuromuscular blockers in neonates and small children.
TGI MH Friday, March 26, 2021: Should a free-standing surgicenter that ONLY uses IV anesthesia be required to stock dantrolene?
Hope you have a great weekend, and see you again Monday.
TGI MH Friday, March 26, 2021
In today’s TGI MH Friday, we’ll try to unpack a thorny issue that lies at the intersection between politics, economics, and patient safety: Should a free-standing surgicenter that ONLY uses IV anesthesia be required to stock dantrolene? And if so, how much is enough? The logical extension of that question is: What is the risk of a patient developing acute MH from a dose of succinylcholine? And finally, what is the likelihood that a patient will be administered succinylcholine during an IV anesthetic? Before we try to answer these questions, some background…and a disclaimer:
Disclaimer: I am currently a member of the Malignant Hyperthermia Association of the U.S (MHAUS), and in the past I have held the positions of Medical Director of the MHAUS hotline, Vice-President for Scientific Affairs, and I have served on the Board of Directors. However, the views I write today are not in any way to be construed as official statements or positions of MHAUS or its officers or other members.
As a patient safety and advocacy organization, MHAUS recommends that “Dantrolene must be available for all anesthetizing locations where MH trigger agents are used.” Triggering agents are commonly considered to be all inhalational anesthetics and succinylcholine. Furthermore, MHAUS recommends that centers stock a minimum of 36 20-mg vials of generic dantrolene (total dose 720-mg), or three 250-mg vials of Ryanodex (total dose 750-mg). These amounts of dantrolene were originally determined by the analysis of MH event data showing that some cases of acute MH required up to or more than 10-mg/kg body weight, and therefore, these total dose amounts would suffice for the majority of average-sized patients that develop MH.

Over the past several decades MHAUS received numerous requests from anesthesiologists at many surgi-centers, as well as from representatives of the Ambulatory Surgery Committee of the American Society of Anesthesiologists, and the Society of Ambulatory Anesthesia, to amend the recommendations for stocking dantrolene in these facilities. The requests were based on three arguments. The first is the assumption that since the incidence of MH susceptibility in the general population is low, and the need for succinylcholine to treat an airway emergency in these centers is unusual, then the likelihood of the above two events happening to the same patient is so low that it renders the cost of stocking dantrolene prohibitively high when compared to its potential usefulness. The second is that accrediting agencies such as The Joint Commission and others have traditionally relied on the expert opinion of patient safety organizations such as MHAUS to determine accreditation criteria. These accreditation organizations, in line with MHAUS recommendations, have taken the stance that surgical facilities must stock dantrolene if they also stock succinylcholine as a requirement to become accredited by the Center for Medicare and Medicaid Services (CMS). The third is that to acquire accreditation, some ambulatory surgery facilities that do not want to incur the cost of dantrolene may choose to not stock succinylcholine, thus putting their patients’ lives at risk in the event of a life-threatening airway obstruction (Joan Rivers?). Of course, this last point does not take into account the availability of sugammadex, which may facilitate the use of high-dose nondepolarizing muscle relaxants to treat life-threatening airway obstruction.
With that as background, let’s try to estimate the risk-benefit relationships for whether we should stock dantrolene when volatiles are not used. First, we need to know the prevalence of MH susceptibility in the general surgical population. This is hard to do because it varies by geographic variation, and we need to exclude those patients that already know about MH susceptibility in themselves or their families. The most conservative (ie, highest) estimate seems to be about 1 in 1,500 patients.
Second, what is the chance that one of your patients who is receiving TIVA will require succinylcholine for an airway emergency? This is impossible to know, as there are no published numbers, so we must rely on one’s notoriously unreliable memory for unpleasant complications. I’ll venture a guess, thinking about laryngospasm in babies for ear tubes and say 1 in 200. But I could be way off in either direction (obviously it’s less in my hands!).
Third, and most important, what is the chance that MH will occur from one dose of succinylcholine? It’s pretty rare, but it’s not zero. The vast majority of MH cases are triggered by a volatile anesthetic agent with or without succinylcholine, but in a small percentage of cases MH appears to be triggered by succinylcholine alone in the absence of a volatile agent. In a report from the University of Toronto MH testing center, 20 of 129 (15.5%) biopsy-proven MH events were triggered by succinylcholine alone. In Europe, 2 of 200 (1%) biopsy proven MH events were due to succinylcholine alone. These numbers are somewhat helpful, but their case control nature introduces plenty of bias as to the real incidence. Think of how many thousands of patients receive succinylcholine in emergency rooms or ambulances and don’t develop MH.
So, armed with those numbers, what is the chance that one of your TIVA patients is unknowingly MH susceptible, and then receives succinylcholine, and then develops MH? Let’s try the math:
1 in 1,500 x 200 x 100 = 1 in 30 million anesthetics! (If we use the conservative Toronto numbers it’s about 1 in 2 million, but these estimates are not based on any reliable data.) Given this rarity, who would buy and stock dantrolene, and re-order it every 3 years, knowing that it will never be used?
In 2016, MHAUS convened a consensus conference of hotline consultants, members of the board of directors, and professional advisory council to discuss the advantages and disadvantages of stocking dantrolene in these types of facilities and whether or not we should change the recommendations. Opinions varied widely and generally fell into one of two approaches. The majority of MH experts believed that as a patient advocacy organization that was originally chartered by MH susceptible patients and has MH susceptible families on the board of directors, the primary responsibility of MHAUS is to protect the health of patients, both known MH susceptibles, and those who will subsequently develop MH, but are as yet unaware of their MH susceptible status. Experts in this group felt that the cost of stocking dantrolene, even if never used, is a relatively small price to pay for the security and confidence of knowing that anesthesiologists can be free to stock and administer succinylcholine for life-threatening airway obstruction without fear of patients developing MH without the only known antidote immediately available. These experts held strong beliefs that one of the missions of MHAUS is to make an MH death a “never event”, and that having an adequate supply of dantrolene wherever triggering agents are administered is crucial to this mission, especially in light of data that demonstrates a convincing relationship between the length of time it takes to administer dantrolene and subsequent patient outcomes.
Other MHAUS experts, however, acknowledged the very low incidence of MH caused by succinylcholine alone and the cost to health expenditures on a more global basis if every surgical facility was required to continuously buy and stock a dantrolene supply that is never used. This group of experts also worried about the health consequences of anesthetized patients if these surgical facilities choose not to stock succinylcholine solely for the reason to avoid the obligation of purchasing dantrolene, and they strongly opposed a recommendation that was not evidence-based. Some experts in this latter group thought that another reasonable option would be to require less than a full recommended dose of dantrolene, reasoning that a “starter” dose would be useful prior to transferring the patient to a full-service medical center.
In the end, the consensus was that the incidence of MH induced by succinylcholine alone was not deemed rare enough to justify the absence of dantrolene wherever succinylcholine may potentially be administered. MHAUS continues to recommend that facilities that stock and have the potential to administer any triggering agent, including succinylcholine without volatile agents, should have a full dose (at least 10 mg/kg corresponding to the estimated size of their patients) of dantrolene immediately available (i.e., the ability to administer dantrolene within 10 minutes of the first sign of MH) in the event that a patient in that facility develops MH, and that organizations that inspect healthcare facilities on behalf of CMS, as well as individual state-based licensing agencies should be the purveyors of decisions that involve healthcare costs to society.
I hope everyone has a relaxing weekend, and for those working for the rest of us, will send out quiet on-call vibes from Philly.
Throwback Thursday, March 25, 2021
Welcome to another edition of PAAD’s Throwback Thursday, where we welcome back eminent professor Myron Yaster. He will revisit a real oldie that established the way in which we dose neuromuscular blockers in neonates and small children.

This article, written almost 40 years ago, remains one of the most important pharmacokinetic and pharmacodynamic papers ever published in pediatric anesthesia. The authors include a who’s who of giants in the fields of anesthesiology and pharmacology, including Dennis Fisher, Don Stanski, Ron Miller, and George Gregory. The authors solved a vexing problem at the time, namely, how to dose non-depolarizing muscle relaxants in infants (and children). The neuromuscular blocking agent (NMBA) used in the study was d-tubocurarine (dTc or curare), a once common NMBA that is no longer used. However, the results remain true for the NMBAs we use today. The problem they solved was a thorny one: in some studies investigators found that infants and children were more sensitive to dTc (compared to adults) and in others they were more resistant. How to square this circle?
Before getting to the results, I urge you to read the METHODS sections of this paper. It is a model of clarity and the techniques used, including a quantitative measure of neuromuscular blockade which can and should be used in our practice today (albeit without the 27-gauge needles and paper recorders!). Measuring dTc concentrations, the authors fitted their results into a 2-compartment, first-order pharmacokinetic model and determined, t1/2 alpha and beta, volume of distribution at steady state (Vdss) and clearance. The paralysis data was then fitted to the kinetic data. The key findings were that the Vdss was much greater in neonates than the other age groups and the plasma concentration required to produce paralysis was much lower. These 2 results basically cancel each other out so that even though less dTc may be required to produce paralysis, the larger volume of distribution requires a higher dose! So, the mg/kg dose would be the same in neonates and adults. These results would be similar for other ionized molecules which remain in the plasma and extracellular fluid (ECF), including all of the NMBAs used in our current practice. As the authors point out, the changes in Vdss mirror the changes in increased ECF in neonates. Another interesting finding was that clearance did not change with age but the t1/2 beta (elimination) was longer in neonates which again is related to the larger Vdss and means that recovery from neuromuscular blockade may be slower in neonates, particularly if multiple doses of NMBA are given.
A final thought to the readers. This study was performed while Dennis Fisher (my former senior resident and good friend) was a fellow at University of California San Francisco. He went on to have a remarkable career as an investigator, author, editor, and consultant and is a testament to his mentors and the institution in which he trained.
PAAD Editor’s Note: I have one funny story about Stanski: when I was in Rochester I was in charge of the visiting professor program. This played a huge role in the trajectory of my career, as I developed relationships with many notable and academically successful people that in many cases carry to this day. But Stanski (then Chair at Stanford) was different – he was serious and a little aloof, and hard to talk with. At that time, I had an old Porsche 911 convertible, which I used to take him back to the airport on a beautiful spring day. I always drove very slow and safe with the VP in the passenger seat, and it gave me time to pepper them with more questions. Upon doing so, Stanski got visibly frustrated and snapped at me: “Enough with the damn questions! How about you put your foot on the gas and let’s see how fast this baby can go!” So I did.
Wednesday, March 24, 2021
It’s a chemo week for me, and I’m tired. So, I’ll take this opportunity to re-run one of the earliest PAADs that you probably haven’t seen, when I had just 3 subscribers, and they were all from my own family. See you again tomorrow for another exciting Throwback Thursday with Guest Reviewer Myron Yaster.
In the February 2021 issue of BJA, Ing et al. chip away at the mystery of neonatal anesthetic induced neurotoxicity. They performed a systematic review and meta-analysis of published prospective studies to determine whether exposure to a single general anesthetic in early childhood (i.e., less than 3 years of age) is associated with long-term neurodevelopmental problems. The meta-analysis included randomized controlled trials as well as non-randomized studies with prospectively collected neurodevelopmental outcomes. After evaluating over 5,000 potential studies, the authors settled on 3 that met the inclusion criteria and used similar outcome scores. They include the PANDA study, the MASK study, and the GAS study. Although none of these individual studies demonstrated differences in neurocognitive or neurobehavioral outcomes in infants exposed to one short general anesthetic compared with controls, the meta-analysis that combined the data from all three (total of about 800 infants) revealed significant differences in the Full-scale Intelligence Quotient and the Child Behavior Checklist, such that infants exposed to the general anesthetic had higher scores (i.e., worse outcomes).
At first glance, this appears to be a significant contribution to the canon of studies that further our understanding of this enigmatic problem, but it’s not that simple. First of all, meta-analyses are notoriously difficult to perform, let alone understand (as a frequent reviewer for the major journals, meta-analyses represented a relatively large proportion of the studies submitted, and very few were eventually accepted for publication). This one suffers from the same kinds of shortcomings as others – disparate methodologies in each of the studies included for analysis. Furthermore, the lower the number of studies included, the more unreliable the results because bias is relatively high, whereas, differences in methodology from a larger number of studies might “wash out” and improve external validity.
But, despite studies such as this, there are more important overarching questions that interfere with our eventual understanding of the neurotoxicity problem, and will influence how we anesthetize small children to minimize neurotoxicity:
1. Within what age range is the brain vulnerable to damage from exposure to anesthetic agents?
2. Which anesthetic agents are responsible for brain damage? Is there a dose-related relationship? Are any anesthetics relatively safer than any others?
3. What is the duration of exposure that increases the risk of brain damage?
4. Are multiple exposures riskier than a single exposure?
5. Does the nature of the surgical intervention make a difference?
6. What are additional predisposing factors? For example, what role is played by comorbidities that require the child to require surgical intervention in the first place?
7. What is the role of other anesthetic-related physiological perturbations such as alterations in blood pressure, temperature, glucose, carbon dioxide, prematurity, socioeconomic status, body temperature, or even head position, to name just a few?
8. What are the specific neurocognitive deficits that are related to anesthetic exposure?
9. Are there any medications that can be administered or substituted in the perioperative period that will mitigate these adverse effects?
After nearly 20 years of intensive study, definitive answers to all of these questions are still lacking.

In the face of this current uncertainty, anesthesia providers should follow these commonsense recommendations:
1. Do not perform elective surgery on children of any age. Strictly speaking, there are no purely elective surgeries that we perform in children younger than adolescence. But there are a number of radiological procedures, especially MRI, that on the surface, appear to have no evidenced-based indication. Young children who are scheduled for MRI with sedation or general anesthesia should be carefully screened with input from the child’s pediatrician or neurologist to clarify the true importance for the scan to be performed during early infancy or childhood.
2. Minimize the duration of exposure to general anesthesia in any one procedure. We all know instances in which the anesthetic exposure time was unnecessarily prolonged when the attending surgeon is finishing a case in another room, or the medical student is taught how to close a wound, or when an inexperienced anesthesia resident is allowed multiple attempts at intubation or IV insertion. That’s not to say that trainees shouldn’t learn on children, but these should be closely monitored and of appropriate duration and attempts. Surgical wait times should be minimized by meticulous planning and organization of multiple procedures by different surgeons. Waiting for the surgeon to finish another child’s procedure in a nearby operating room (or building!) is never appropriate.
3. Use regional anesthesia whenever possible. There aren’t many surgical procedures performed in young infants that are not amenable to using local anesthesia to reduce intraoperative anesthetic requirements. When feasible, local anesthesia should be used from the outset of the procedure, not just for the purpose of reducing postoperative pain. In an off-shoot of the GAS study, McCann et al. demonstrated that spinal anesthesia for hernia repair resulted in less hypotension when compared with general anesthesia.
4. Keep all physiologic parameters well within “normal” ranges. Yes, of course I understand that everyone’s definition of “normal” may differ, but in general, be conservative with expectations for indices such as the lowest acceptable intraoperative blood pressure, hemoglobin, glucose level, temperature, and end-tidal CO2 values.
5. Keep the child’s head in a neutral position. There is evidence that lateral head rotation may diminish blood flow in the jugular veins or carotid arteries. There’s no clinical reason why this can’t be accomplished during a surgical procedure that doesn’t involve one side of the head or neck.